Target audience
The course is specifically designed for laboratory staff responsible for implementing diagnostic techniques to detect bluetongue virus (BTV). The course can also be useful to anyone with a general interest in learning about the pathogenicity, clinical disease, epidemiology, and control of the virus.
Requirements and qualifications
Access to a computer with a reliable internet connection is essential. The course is prepared in English (UK).
Course description
This eLearning course designed in collaboration with subject matter experts in the BTV WOAH reference laboratory at Pirbright, is intended to broaden participants understanding of bluetongue, and the virus which causes it. The course specifically focuses on information that would be useful for vets or laboratory staff undertaking laboratory diagnostic testing of BTV.
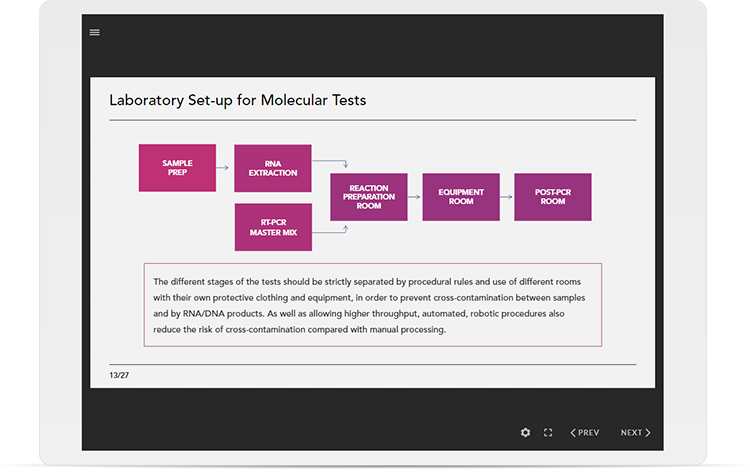
Learning outcomes
Divided into 10 individual modules, you will be able to:
- Discuss the history and impact of BTV.
- Describe the pathogenesis and spread of BTV including incubation and persistence in carrier animals.
- Recognise the clinical signs of BTV and compare to similar diseases for differential diagnoses.
- Explain the importance of laboratory diagnosis for BTV including which samples and tests are important to confirm disease and for surveillance.
- Describe laboratory diagnostics including molecular, virological, and serological techniques, as well as next generation sequencing.
- Outline methods for sample dispatch and receipt.
Course modules
The course comprises of the following modules:
| Module title | Duration (minutes) | No of Questions | |
|---|---|---|---|
| 1. | Introductory module; covering the BTV, the disease and the work of the BTV Reference Laboratory at Pirbright | 30 | 10 |
| 2. | Pathogenesis and spread of BTV | 20 | 5 |
| 3. | Clinical diagnosis of BTV | 45 | 5 |
| 4. | Introduction to BTV laboratory diagnosis | 20 | 5 |
| 5. | Sample dispatch and receipt | 40 | 4 |
| 6. | BTV virus detection | 30 | 6 |
| 7. | BTV serology | 30 | 6 |
| 8. | Further characterisation of BTV by sequencing | 20 | 3 |
| 9. | An introduction to quality assurance | 15 | 7 |
| 10. | Biosafety and biosecurity in the BTV laboratory | 90 | 17 |
Next steps
Contact Pirbright Training for portal access details.

