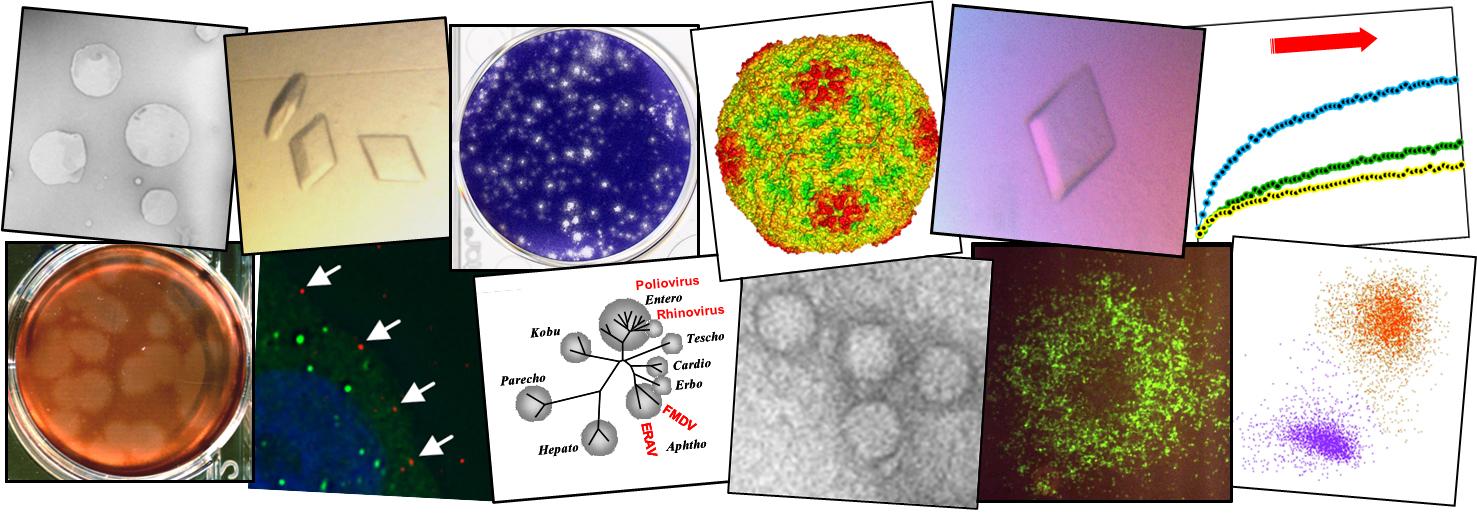
The cellular chaperone heat shock protein 90 is required for foot-and-mouth disease virus capsid precursor processing and assembly of capsid pentamers
Productive picornavirus infection requires the hijack of host cell pathways to aid with the different stages of virus entry, synthesis of the viral polyprotein and viral genome replication. Many picornaviruses, including foot-and-mouth disease virus (FMDV), assemble capsids via the multimerisation of several copies of a single capsid precursor protein into a pentameric subunit which further encapsidates the RNA. Pentamer formation is preceded by co- and post-translational modification of the capsid precursor (P1-2A) by viral and cellular enzymes, and the subsequent rearrangement of P1-2A into a structure amenable to pentamer formation. We have developed a cell-free system to study FMDV pentamer assembly using recombinantly expressed FMDV capsid precursor and 3C protease. Using this assay, we have shown that two structurally different inhibitors of the cellular chaperone heat shock protein 90 (hsp90), impeded FMDV capsid precursor processing and subsequent pentamer formation. Treatment of FMDV permissive cells with the hsp90 inhibitor prior to infection reduced the endpoint titre by more than ten-fold while not affecting the activity of a sub-genomic replicon indicating that translation and replication of viral RNA were unaffected by the drug.IMPORTANCE Foot-and-mouth disease virus (FMDV), of the Picornaviridae family is a pathogen of huge economic importance to the livestock industry due to its effect on the restriction of livestock movement and necessary control measures required following an outbreak. The study of FMDV capsid assembly, and picornavirus capsid assembly more generally, has tended to be focused upon the formation of capsids from pentameric intermediates, or the immediate co-translational modification of the capsid precursor protein. Here we describe a system to analyse the early stages of FMDV pentameric capsid intermediate assembly and demonstrate a novel requirement for the cellular chaperone hsp90 in the formation of these pentameric intermediates. We show the added complexity involved for this process to occur which could be the bases for a novel antiviral control mechanism for FMDV.