Avian leukosis virus p15 interacts with interferon regulatory factor 7 to antagonize the cGAS-STING signaling pathway and promote viral replication
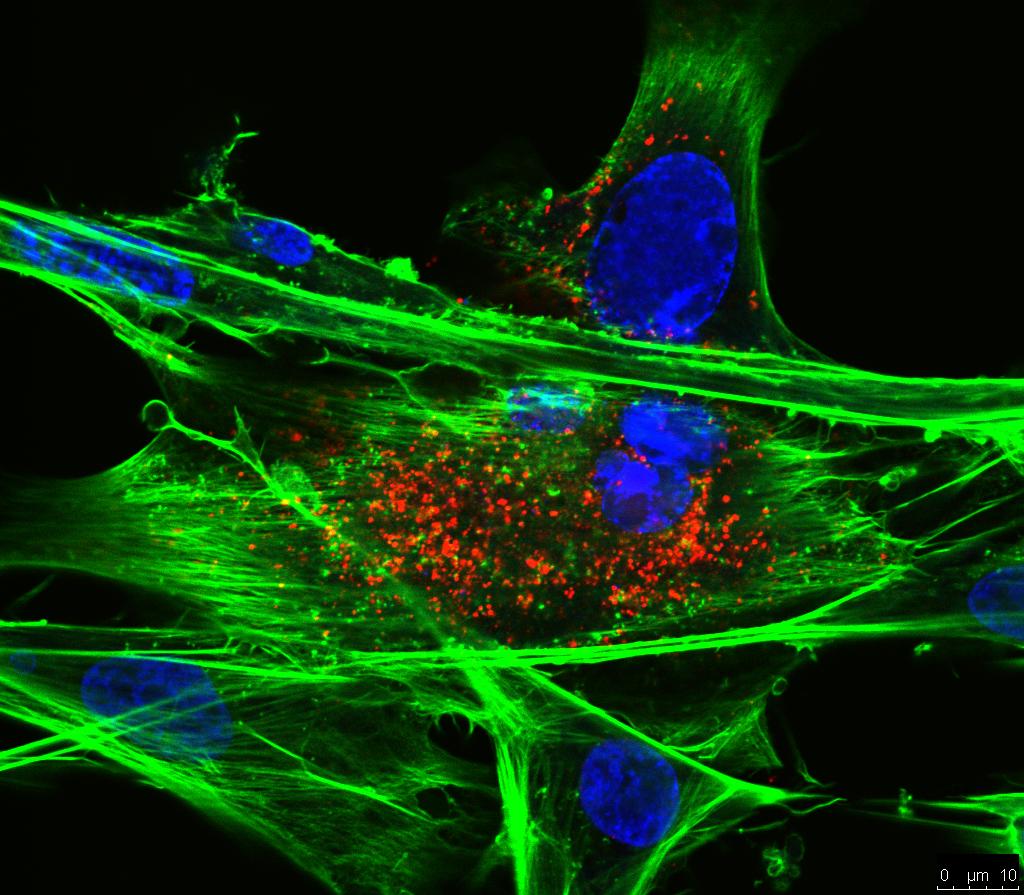
Cyclic GMP-AMP synthase (cGAS) recognizes viral DNA within the cytoplasm and initiates innate antiviral response through the cGAS-STING pathway. However, viruses have evolved diverse strategies to counteract the cGAS-STING signaling pathway. Avian leukosis virus subgroup J (ALV-J), an avian oncogenic retrovirus, can antagonize host innate immune responses and cause immunosuppression to develop tumors. In this study, with a functional screen, we identified the p15 protein, a protease encoded by ALV-J, inhibiting the cGAS-STING signaling pathway and IFN-β production to promote virus replication. This inhibitory effect is associated with the enzymatic active site of p15. Further study demonstrated that the p15 protein interacted with the DBD domain of interferon regulatory factor 7 (IRF7), inhibiting the dimerization and nuclear translocation of IRF7 but not the phosphorylation of IRF7, resulting in the suppression of IFN-β production. Consistent with these results, small-interfering RNA targeting p15 in ALV-J infection induced more IFN-β in DF-1 cells compared with control RNA. Taken together, these results verified that ALV-J-encoded p15 protein plays a key role in evading the cGAS-STING pathway, which improves our understanding of the virus-host interaction in ALV-J evading host innate immunity and may contribute to developing novel drugs against ALV-J infection.
Importance: Among all subgroups, avian leukosis virus subgroup J is one of the most pathogenic, capable of inducing severe malignant tumors and immunosuppressive effects on the infected host. Although there have been some reports on the immunosuppressive mechanisms of ALV-J, the immune evasion mechanism mediated by ALV-J-encoded proteins remains largely unknown. In this study, we found that p15 interacted with IRF7 and inhibited the dimerization and nuclear translocation of IRF7, resulting in the suppression of the expression of IFN-β. Of note, the results demonstrated that the enzymatic active site of p15 (37D, 38S) plays a crucial role in this process. Our findings revealed that p15 enhanced ALV-J replication by inhibiting the cGAS-STING signaling pathway, which highlights the possibility of p15 as a potential drug target.