A grant worth £2,359,553 was awarded to The Pirbright Institute’s Dr Simon Graham to lead an international team developing an inexpensive, safe and effective vaccine to protect pigs against Nipah virus (NiV). The project ‘A Nipah vaccine to eliminate porcine reservoirs and safeguard human health’ was funded by UK Department of Health and Social Care through the Innovate UK SBRI Vaccines for Global Epidemics – Clinical competition.
Our partners
This grant is allowing scientists from Pirbright and partners in the UK, Australia, Malaysia and India to develop a prototype vaccine that will aid the prevention and control of NiV outbreaks. The project partners are:
- Dr Simon Graham, Dr Dalan Bailey, Dr Elma Tchilian, Dr Rebecca McLean, Dr Miriam Pedrera and Miss Nazia Thakur, The Pirbright Institute, UK
- Dr Teresa Lambe and Prof Sarah Gilbert, Jenner Institute, University of Oxford, UK
- Dr Glenn Marsh, CSIRO Health and Biosecurity, Australia
- Prof Paul Young, Dr Keith Chappell and Dr Daniel Watterson, University of Queensland, Australia
- Dr Li-Yen Chang, University of Malaya, Malaysia
- Prof Nagendra Nath Barman, Assam Agricultural University, India
- Dr Mustafizur Rahman, iccdr,b
- Dr Mercedes Mouriño, Zoetis
Our aims
The project will provide:
- The first head-to-head comparison of the efficacy against challenge of recombinant NiV vaccines in a target species (pigs);
- The first examination of the ability of vaccines to block NiV transmission and
- The first systematic analysis of T and B cell immune responses in a target host to NiV vaccines to define correlates for protective immunity.
Consequently, we will be well placed at completion of the project to proceed to the next stages of development of a commercial NiV vaccine for use in pig populations within the low and middle-income country setting. By defining correlates of protection in pigs, this project will support human vaccine licensure under the Animal Rule as efficacy trials in humans are unlikely to be possible.
- A Target Product Profile (TPP) has been created for a Nipah virus vaccine for use in pigs.
Our research
In this project we shall systematically analyse in pigs the immunogenicity and protective efficacy of three NiV vaccine candidates:
- A computationally codon optimized NiV G protein subunit with adjuvant,
- A molecular clamp stabilised NiV F protein subunit with adjuvant,
- A replication-deficient simian adenovirus (ChAdOx1) vectored NiV G protein.
In addition, for the first time we shall examine the durability of the response, determine the efficacy of a ‘one-shot’ immunisation regime and establish whether the vaccines prevent NiV transmission.
Our impact
NiV causes a severe and often fatal neurological disease in humans. Whilst fruit bats are considered the natural reservoir, NiV also infects pigs and may cause an unapparent or mild disease. Direct pig-to-human transmission was responsible for the first and still most devastating NiV outbreaks in Malaysia and Singapore in 1998-99, with nearly 300 human cases and over 100 fatalities. Pigs therefore play a key role in the epidemiology of NiV by acting as an ‘amplifying’ host. The outbreak in Singapore ended with the prohibition of pig imports from Malaysia and the Malaysian outbreak was ended by culling 45% of the country’s pig population. The development of an inexpensive, safe and efficacious vaccine for use in pigs to protect against NiV infection and transmission will reduce the risk to public health (Figure 1). The vaccine will also reduce the major risk NiV poses to both the nascent developing pig industries, as well as to the livelihoods of poor livestock keepers in South and Southeast Asia. The project will also provide a solid basis for the further evaluation of the vaccine for protection of humans against NiV infection.
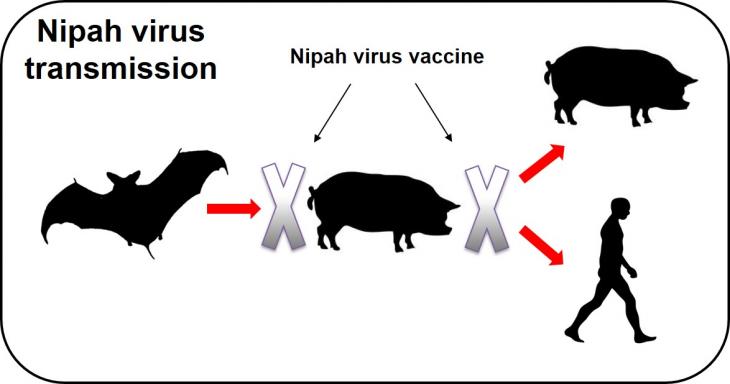
Figure 1: Nipah virus transmission could reduce virus dissemination between pigs and also prevent transmission to bats (NiVs natural reservoir) and humans.

